NEWS AND EVENTS
NEW PUBLICATION FROM THE LABORATORY OF BIOCHEMISTRY DESCRIBES THE MECHANISMS OF ALLOSTERIC INHIBITION OF INSULIN-REGULATED AMINOPEPTIDASE
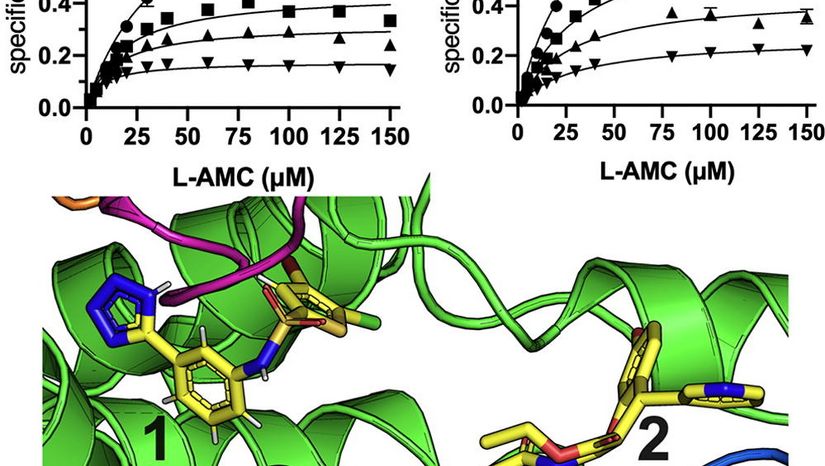
Congratulations to Anastasia Mpakali and co-authors Ioanna Barla, Liying Lu, Karthik Ramesh, Nikos Thomaidis, Lawrence Stern and Petros Giastas for this recent publication in the Journal of Molecular Biology. In this paper, we describe the mechanism of two commonly used inhibitors of Insulin-Regulated Aminopeptidase, an enzyme with several important biological functions and a potential pharmaceutical target. Using a combination of kinetic, biophysical and X-ray crystallographic techniques, we find, that, despite previous hypotheses, these inhibitors do not target the active site, but rather, target allosteric sites and stabilize distinct conformations of the enzyme. Furthermore, we find that the commonly used inhibitor HFI-419 is substrate-specific and has very low potency for an important physiological IRAP substrate, the hormone oxytocin. Our findings demonstrate how conformational restriction by allosteric inhibitors can be potentially used to target distinct biological functions of this enzyme.
https://www.sciencedirect.com/science/article/pii/S0022283624000159
