BIOCHEMISTRY OF THE NERVOUS SYSTEM (DR. E. EMMANOUILIDOU)
Secretion and degradation of neuronal proteins involved in neurodegenerative diseases
The central focus of research is the molecular mechanisms of secretion and degradation for the protein α-synuclein, which is of particular importance in the pathobiology, pathology propagation and disease progression of Parkinson’s Disease and other related neurodegenerative conditions collectively called synucleinopathies. In this field we have contributed with two significant discoveries: (i) that part of cytoplasmic α-synuclein is secreted in association with exosomes, small vesicles of endocytic origin that have been implicated in cell-to-cell communication, and (ii) that the inter-neuronal mechanism for α-synuclein secretion in vivo in the striatum is tightly controlled by the neurotransmitter GABA.
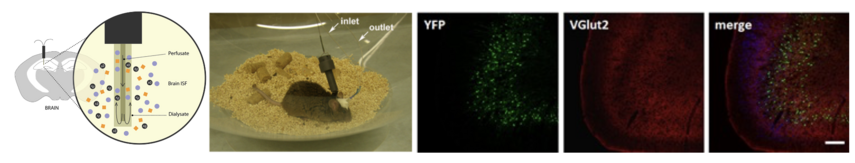
Our work is performed in a cellular level in vitro using cells in culture and brain tissue but also in vivo in the context of a cellular network (i.e. neural circuits, neuron-astrocyte network) using mouse models. Towards this direction, we have established an in vivo brain microdialysis approach coupled with an ultra-sensitive in-house ELISA to monitor and pharmacologically manipulate α-synuclein secretion in mouse brain parenchyma.
To date the major research directions of the lab are:
(a) the identification of the oligomeric α-synuclein species that burden the proteasome degradation system;
(b) the interactions of α-synuclein and its oligomeric forms with free fatty acids;
(c) the elucidation of key molecules that trigger or mediate the secretion of α-synuclein with emphasis on the operation of voltage-gated Ca2+ channels and ATP-dependent K+ channels;
(d) the role of α-synuclein in neuroinflammation with emphasis on the effects of α-synuclein assemblies in microglia and astrocyte activation;
(e) the development of sensitive and specific assays as tools to evaluate the potential use of certain biomolecules as biomarkers for the diagnosis or staging of human disease.